It seems that naturally curly folks have a love/hate relationship with glycerin. There is a lot of information, and misinformation, about this seemingly simple substance. I thought I’d shed some light on the chemical makeup of glycerin and how it affects curly hair specifically.
So, what is it
Glycerin is an alcohol, known also by the chemical names glycerol and 1,2,3, propane triol. It can be obtained via hydrolysis of naturally occurring plant or vegetable fats (triglycerides”> or via chemical synthesis from petrochemicals. It is a conditioning alcohol similar to other conditioning alcohols, but it has three very hydrophilic hydroxyl (-OH”> groups as opposed to a single one. Because of this, glycerin is far more water soluble than some of the other conditioning alcohols such as lauryl alcohol and cetearyl alcohol.
Glycerol has been used for many years in cosmetics and personal care, and has been found to be a remarkable moisturizer for the skin and hair. In fact, new studies have shown that it has amazing abilities to actually aid in repair and regeneration of skin cells.

Figure 1
Chemical structure of glycerol
Glycerol obtained from naturally occurring fats is a product of chemically breaking down triglyceridesinto their fatty acid components. Triglycerides are fat molecules comprised of a glycerol backbone with three arm-like appendages of fatty acids bound to the glycerol via an ester linkage. These three fatty acid chains can all be the same exact molecule (such as stearic acid”>, or can all be different from one another (one stearic, one lauric, one palmitic – for example”>. Hydrolysis of the ester bond results in the production of glycerol and three fatty acid molecules.
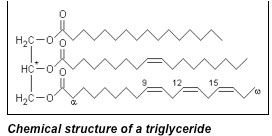
Figure 2
Glycerin can come from multiple natural sources because triglycerides can be derived from animals or vegetables. Some examples of vegetable sources would be coconut oil and shea butter. Typically, if you are purchasing glycerin, the label will say if it is from a vegetable source of glycerin. However, a multi-ingredient finished product may not disclose the source of glycerin (animal, vegetable, or synthetic”> unless it is a marketing point for the brand.
Synthetic glycerin has received some publicity as being a potential health hazard, and many consumers prefer to avoid it. It is typically produced from the starting material epichlorohydrin, which is a toxic chemical that is classified as a probable carcinogen. Of concern is the presence of trace remnants of epichlorohydrin or another potentially carcinogenic contaminant such as 1,4 dioxane. So, while synthetic glycerin provides the same benefits to your hair as glycerin derived naturally, there may be sufficient risks associated with it to warrant choosing only vegetable or animal-derived glycerin.
Properties of Glycerin:
Glycerin is an alcohol and is soluble in water and also in other alcohols. It is insoluble in oils, but is able to dissolve many oils and can be used as an emulsifier for adding oils into a formulation. In its pure form, it is odorless and colorless, but has a sweet taste. It has a thick viscosity and is clear, so it is frequently added to formulations for its moisturizing and viscosity-modifying properties. Adding it to a formula is an inexpensive way to impart a thick, velvety texture to a product, a property typically valued by consumers. It is a highly effective moisturizer and humectant for skin and hair. Its thick viscosity and high boiling point are what make it an effective curl-definer and frizz minimizer (in the right climate”>.
Glycerin is a relatively small molecule compared to many moisturizers, and it contains three hydroxyl groups. This high molecular density of hydrophilic groups makes it an extremely hygroscopic molecule that absorbs water from its surrounding environment. It does this to such a high degree that it will raise a blister if applied in an undiluted state to the skin. If it were applied to hair in such a concentrated state, it could strip all of the moisture from the interior of the hair.
However, when used in a diluted form, glycerin can be a great moisturizer and humectant for the hair. Care should be taken to use it in environments of moderate humidity. If the climate is very hot and humid, glycerin will absorb a lot of moisture from the air and cause the hair to swell, raising the cuticle and disrupting curl pattern, creating coarse, frizzy hair. In weather that is extremely dry, glycerin will seek out moisture from your hair and actually dehydrate it, which can cause damage and breakage. Read more about this.
Concerns and precautions
Many heat-styling techniques can generate sufficient heat to boil the water inside the hair shaft, which can cause terrible breakage. One way to prevent or minimize this problem is to coat the hair with an emollient that seals in the moisture and that does not transfer the heat from the appliance to the hair as readily. Unfortunately, glycerin conducts thermal energy pretty efficiently (it transfers heat readily to the hair”>, especially when compared to silicones, proteins, and polyquaternium conditioning ingredients. For that reason, use glycerin sparingly and in combination with a more insulating and protective moisturizer when using any sort of heated drying or styling treatments.
One of the biggest concerns I have seen expressed about glycerin is that it might remove or strip color from the hair. Many people with chemically dyed hair avoid it almost unilaterally. The truth of the matter is that glycerin is a reasonably good solvent for many types of molecules. It can dissolve and grab unbound dye molecules that are easily accessible near the exterior of the hair.
This is relevant in a few different cases. Users of permanent chemical dyes should exercise some caution in the initial days following processing. Hair that has been freshly colored with a permanent chemical dye is susceptible to loss of some colorant molecules because the cuticle may not have completely re-sealed. However, it is not cause for concern after the first washing because the colorant molecules have penetrated into the cortex of the hair shaft, the cuticle is sealed, and any excess on the surface is washed away.
Users of semi-permanent dyes or colors notorious for being short-lived (such as shades of red”> may be wise to be extremely cautious regarding the use of glycerin in their hair. This is because these types of dye molecules are too large to penetrate the hair and reside predominantly on the exterior of the hair shaft. Glycerin can easily dissolve and remove these colors and accelerate the inevitable fading process.
To Sum it Up
Glycerin is a water-soluble conditioning alcohol and is an extremely effective moisturizer and humectant. This means that all the usual things to be aware of regarding humectants, curly hair, and the weather are applicable when using glycerin.
Its viscosity and clarity make it a great ingredient to give definition to curls and to smooth the fly-away hair.
Use it in combination with another conditioning agent if planning to use heat-styling methods.
Do not use glycerin right after using a permanent dye on the hair, and consider avoiding it if you use semi-permanent or red color on your hair.
